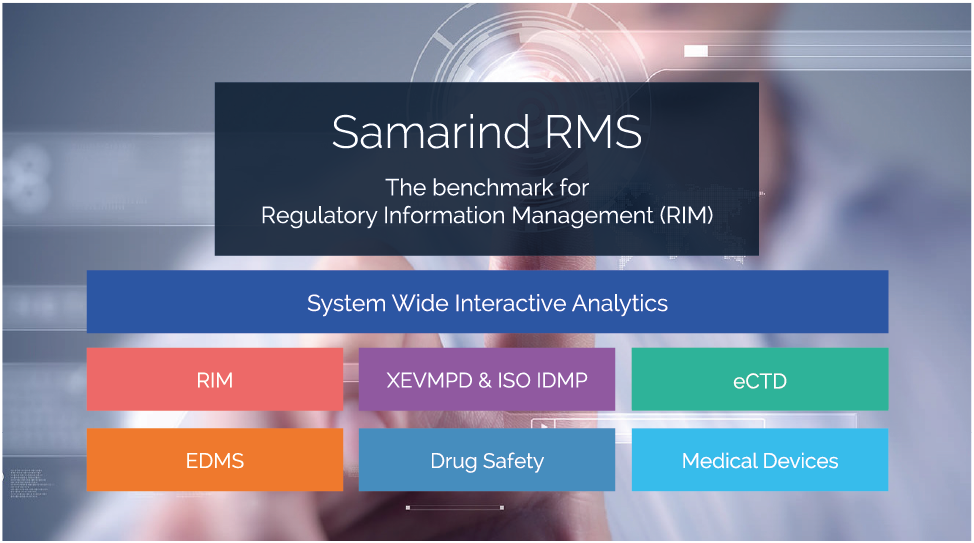
The Samarind RMS suite is a fully integrated software solution that has been purpose built to mirror the processes associated with acquiring and maintaining product licenses.
Samarind RMS provides a smarter way to manage your Medical Product Information. Using Samarind RMS, you only need to enter data once and can reuse it as many times as required. For example an ‘INN’ can be entered once and reused for all related IMA submissions, renewals, variations, PSURs and XEVMPD submissions. This concept applies to all key data held within the system and is proven to streamline workflows and help to increase data quality.
Our pragmatic approach to systems design and implementation means that our customers can manage their licenses smoothly and efficiently, safe in the knowledge that our single-place-of-truth™ approach for regulatory affairs professionals delivers a complete end-to-end system.
Our features include:
- A secure Regulatory Information Management (RIM) system with planning, tracking, automated alerts and comprehensive reporting facilities
- An electronic document management system (EDMS) with version control, template creation and the ability to link to external document management systems such as Documentum™ or SharePoint™
- An optional eCTD module for dossier creation and maintenance (VNeeS & NeeS are also supported)
- An optional EVMPD module for automated maintenance of data required by the EMA’s extended medicinal product dictionary, XEVMPD with a clear roadmap for IDMP
- A Med Info addition, for quick and easy logging of medical information queries, with links to the associated products elsewhere in the system as necessary
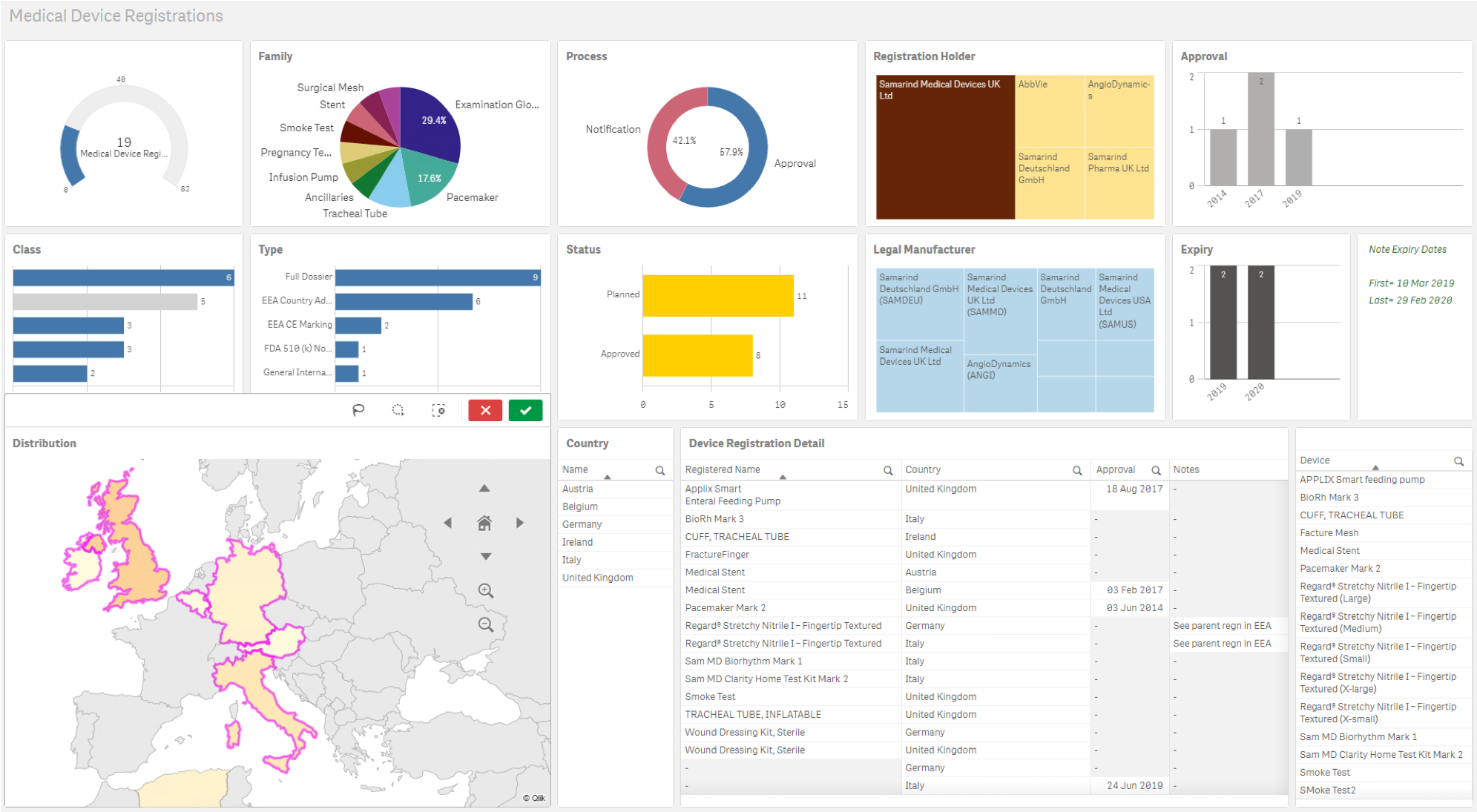
- Medical device registration solution with project management support and FDA UDI generation
- A Drug Safety module, handling Pharmacovigilance requirements
- Project Management Solution, a managers tool linking your tasks & data
Key Benefits
|
Download a Fact SheetSamarind RMS Overview |
Regulatory Information Management
The core of Samarind RMS is its regulatory information management database. This has been carefully structured to allow you to accurately record and maintain all your medicinal product information throughout its lifecycle.
The Samarind RMS Windows software application provides the security, flexibility and ease of use that your regulatory affairs team needs to meet its regulatory and commercial obligations.
- At-a-glance visibility of all the products in your portfolio;
- A secure central repository of all your product documentation, from SmPCs and PILs to PSURs and business contracts ;
- Sophisticated administrator-configurable role-based user permissions, for flexible security;
- Event tracking for drug licensing applications, variations, PSURs and renewals;
- Record information about key partners e.g. API manufacturers and QC Testing sites;
- Time stamped historical audit of all previous submissions and variations;
- Dashboard KPI's & system generated alerts at key stages;
- All data is contained within the same SQL database for security, robustness and performance;
- Extremely flexible and user friendly search and reporting facilities.